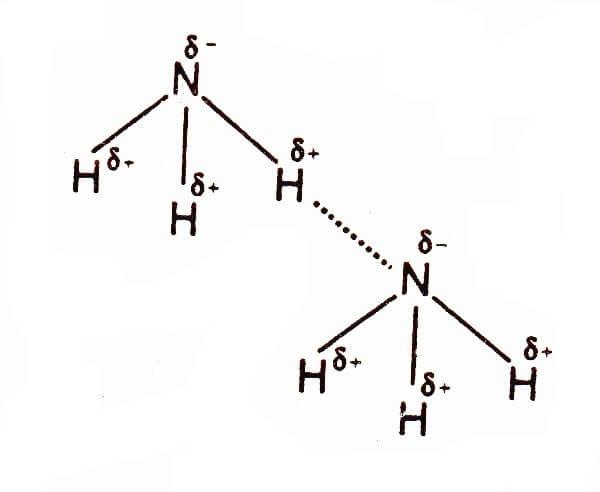
It is a commercially important compound and is also a common nitrogenous waste. It is produced naturally as well as through human activities. Ammonia can be hazardous in concentrated form. It is readily soluble in water. In this article, we will study whether NH3 molecules have hydrogen bonds. So, let’s get started. So, does NH3 have hydrogen bonding? Yes, NH3 forms hydrogen bonds. Hydrogen bonding is the intermolecular forces acting between ammonia molecules. Due to the electronegativity difference between the nitrogen atom and hydrogen, a partial negative charge develops on nitrogen while a partial positive charge develops on the hydrogen atom. These charges are responsible for pulling the nearby NH3 molecules closer and forming hydrogen bonds with them. Let us check this in a detailed way. Stay connected.
Why does NH3 form Hydrogen Bonds?
Hydrogen bonds are formed when a hydrogen atom is attached to an atom with high electronegativity. Looking at the NH3 molecule the electronegativity of nitrogen is 3 while that of the hydrogen atom is 2.2 thus, creating a huge negative difference between the two atoms. Also, as the nitrogen atom has 5 electrons in its valence shell, after bonding with three hydrogen atoms, it is still left with one lone pair of electrons. Due to the high electronegativity difference between the two atoms the nitrogen atom pulls the shared pair of electrons towards itself owing to which a partial negative charge develops on the nitrogen atom. As the electrons are pulled away from the hydrogen atom it acquires a slight positive charge. In addition, the nitrogen atom also has a lone pair of electrons which makes the negative forces around this atom rather prominent due to which the hydrogen atoms of the other molecules of ammonia, having a slight positive charge, are attracted towards the nitrogen atom.
These interaction forces between the nitrogen atom of one molecule and the hydrogen atom of another ammonia molecule result in the formation of hydrogen bonds between different molecules of ammonia. Therefore, the electronegativity difference between hydrogen and nitrogen atom, and the availability of lone pair with nitrogen are responsible for the formation of intermolecular hydrogen bonding in NH3 molecules.
What is Hydrogen Bonding?
A hydrogen bond is an attractive force that can occur between certain molecules when hydrogen is attached to more electronegative atoms like nitrogen, oxygen, and fluorine. These forces are weaker than the usual chemical bonds formed between the atoms viz. ionic and covalent bonds. The strength is gauged based upon the energy required to break the bonds which is much lower in the case of hydrogen bonds. However, if large numbers of hydrogen bonds are present in a compound it may result in very strong forces. The hydrogen bonds are formed as a result of unequal charge distribution inside a molecule, a property of molecules often referred to as polarity.
For example in the case of water or ammonia, the oxygen and nitrogen atoms, respectively, are more electronegative than the hydrogen atoms attached to them. The higher electron affinity of nitrogen or oxygen atom results in the development of a partial negative charge on the more electronegative atom and a partial positive charge on the other atom. As the oppositely charged ends of the two molecules of the same compound come closer to each other it results in the formation of weak inter-molecular attraction forces between these molecules which is known as hydrogen bonding. These bonds are mainly formed due to the electrostatic attraction between the molecules, and become more prominent as the distance between the molecules minimizes. Therefore, the number of hydrogen bonds increases as the distance between the molecules decreases. This is the reason that water has the maximum number of hydrogen bonds in solid form and almost no hydrogen bonds when it is present as a vapor. Hydrogen bonds are also important in the case of proteins, nucleic acids, and other important biological molecules.
Does NH3 form Hydrogen Bonds with Water (H2O)?
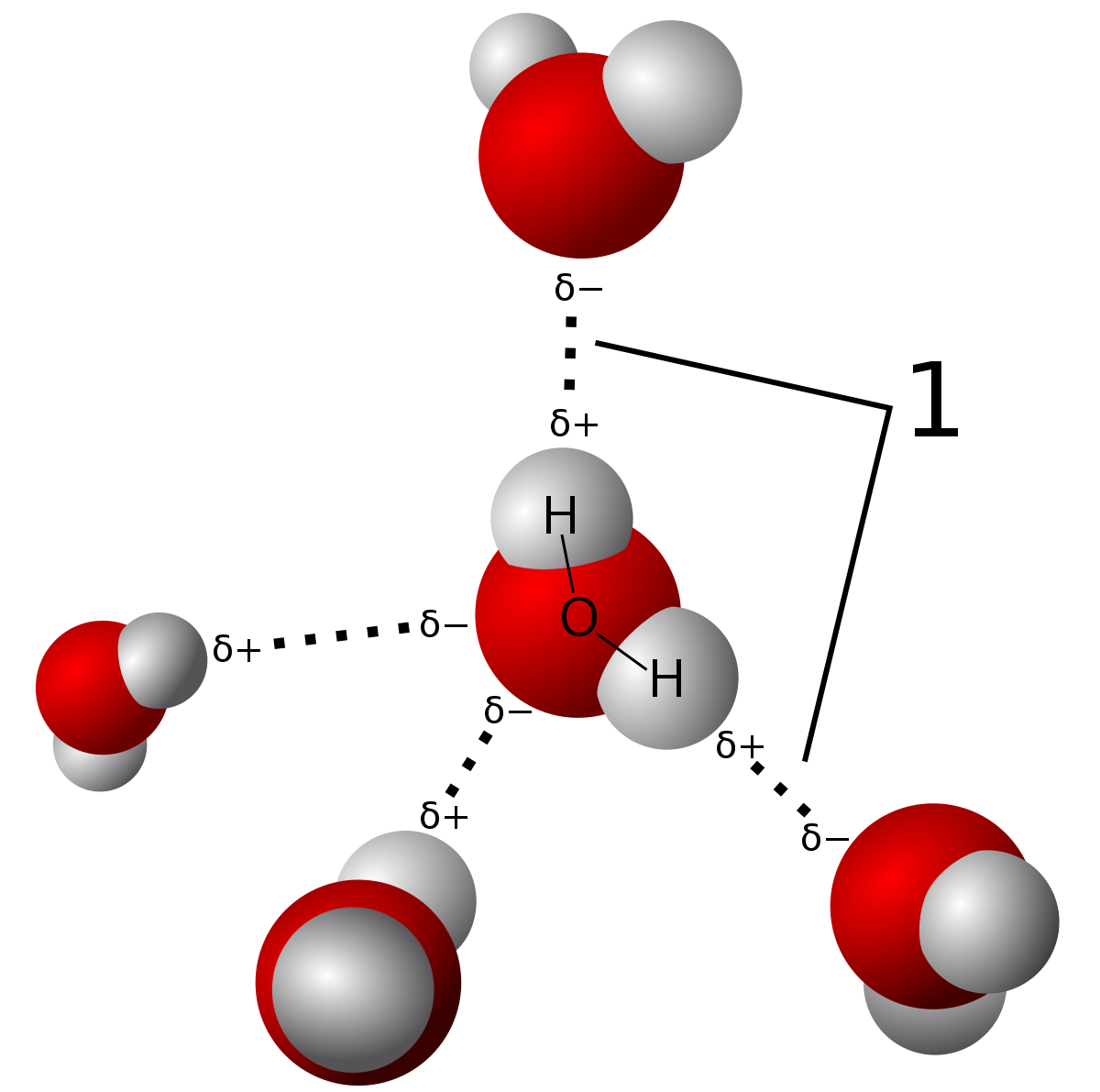
As discussed in the previous section hydrogen bonds are formed between the molecules of a compound in which hydrogen is bonded with a more electronegative atom such as nitrogen, fluorine, and oxygen. In the case of water molecules, the oxygen atom being more electronegative attracts the bonded pair of electrons towards itself and acquires a partial positive charge thus, implanting a partial negative charge on the hydrogen atom. Also, the oxygen atom has six valence electrons which means that after forming covalent bonds with the two hydrogen atoms it is still left with two lone pairs of electrons that are responsible for creating a cloud of negative charge around the oxygen atom. This negative charge is responsible for attracting the hydrogen atoms of other molecules of water and the development of hydrogen bonds.
Therefore, both ammonia and water molecules are bonded through hydrogen bonds. However, the hydrogen bonding in water is stronger in comparison to ammonia as oxygen is more electronegative than nitrogen and also, has two lone pairs of electrons. In the case of the NH3 molecule, there are not enough lone pairs available to satisfy all the hydrogen atoms carrying a partial positive charge, and hence, ammonia easily forms hydrogen bonds with the water molecules when these are mixed together. Actually, hydrogen bonding is the reason why ammonia dissolves so easily in water.
Why does NH3 form Hydrogen Bonds while PH3 does not?
Both nitrogen and phosphorous are group 15 elements and have five electrons in their valence shells and also they both react with three atoms of hydrogen forming their respective compounds viz. Phosphine for phosphorous and ammonia for nitrogen. However, the molecules of ammonia are bonded through hydrogen bonds but such bonding does not occur in the case of phosphorous. The reason for this is the large atomic radius of phosphorous. Looking at their electronic configuration: Nitrogen (N): [He] 2s22p3 Phosphorous (P): [Ne] 3s23p3 As evident from their electronic configuration of nitrogen, the electrons are located close to the nucleus due to which the atom exhibits high electronegativity which is responsible for the intermolecular hydrogen bonding in the case of ammonia atoms. On the other hand, the electrons of the phosphorous atom are located far away from the nucleus in the third orbital due to which the electrons are loosely bound, and hence, the phosphorous atom does not have high electronegativity. Therefore, the phosphorous atom is unable to exert high electron affinity and thus, cannot pull the electrons in the PH3 molecule towards itself. As no partial charges are developed on the atoms there are no electrostatic forces or negative charges available to attract the atoms from other molecules and hence, no hydrogen bonds are formed in the case of phosphine.
Why is Hydrogen Bonds formed?
The main reason for the formation of hydrogen bonds is the electronegativity difference between the hydrogen atom and the connected atom viz. nitrogen, oxygen, or fluorine. The atom with greater electron affinity pulls the electron away from the hydrogen atom causing it to develop a slight negative charge while the hydrogen atom attains a slight positive charge. Also, the atoms that form hydrogen bonds also have lone pair of electrons attached to them which further enhances the negative charge around the atom due to which the oppositely charged atoms from other molecules are attracted towards them and due to electrostatic forces result in the formation of hydrogen bonds. Although weak in nature but hydrogen bonds are still able to hold the molecules of the compound together.
Properties of NH3
A few important properties of hydrogen atoms are listed in the table below:
Uses of NH3
Some of the important uses of ammonia are given below: • Almost 88% of the total world production of ammonia is used in fertilizers. • Ammonia is used for the production of most nitrogenous compounds such as Nitric acid, hydrazine, hydrogen cyanide, urea, etc. • 5-10% by weight solutions of ammonia with water are used as household cleaners especially for glasses. • It is also used in fermentation industries for nitrogen-consuming microbes. • Ammonia is also a strong antiseptic due to which it is used as an antimicrobial agent. • Ammonia is also emerging as a promising fuel source as it has almost one-third of the energy density of diesel. • It is also used as a gas in air-conditioning equipment and as refrigerant gas. • Ammonia is also used in the paper, leather, and rubber industries. • It is also used in the treatment of wastewater.
Conclusion
Ammonia molecules form intermolecular hydrogen bonding due to the high electronegativity difference between the nitrogen and hydrogen atoms. Being more electronegative nitrogen atom exerts high electron affinity attracting the shared pair of electrons towards itself and acquiring partial negative charge and causing hydrogen to develop a partial positive charge. The partial negative charge of nitrogen along with the lone pair of electrons located over this atom causes the positively charged atoms of the other molecules to develop weak intermolecular attractions that are known as hydrogen bonds. Ammonia is soluble in water due to the formation of hydrogen bonding between these molecules. PH3 does not form a hydrogen bond unlike the NH3 molecule as the atomic radius of phosphorous is quite large in comparison to nitrogen and, hence, is unable to exert comparable electronegativity. I hope I made you understand the detailed reasons for hydrogen bonding in NH3 molecules. If you liked the article, do share it with your friends. Thanks in advance!!